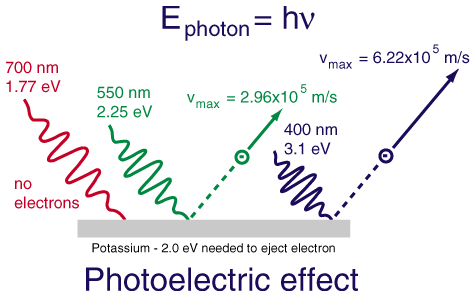
What Is the Energy of a Photon of Green Light
11 Why does chlorophyll not absorb green light. The energy of a photon is determined by the light frequency f.
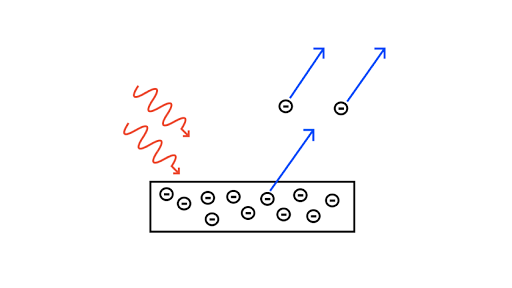
Photoelectric Effect Article Photons Khan Academy
This stimulates the production of adenosine triphosphate or ATP if you recall your high school science class.

. What is the energy of a photon of green light with a frequency of 576 x 10 14 s 1. Lets use the equation below to determine the answer. First we convert the wavelength to the frequency of the radiation and then use the frequency to calculate the energy per photon.
An electron volt is the energy required to raise an electron through 1 volt thus a photon with an energy of 1 eV 1602 10 -19 J. E 0030 x 1017J. Therefore the energy of a photon of green light is 454 x 10¹⁹ J.
How Many Photons Are Contained In A Flash Of Green LightSo the number of photons is 4991023 photonsJul 19 2021What is the photon of green lightThe energy of a photon of green light is 381019 J 38 10 19 J. E 66261034 3108 650109. Expert Answer Solution for Green light has a wavelength of 50 x 102 nm.
To find the energy in joules of one photon of green light. 5 Which color has the highest energy Why. In the visible spectrum red has the longest wavelength meaning.
If we consider the light wave-like then the frequency indicates how often the light wave oscillates per second. Hz is hertz or reciprocal seconds. What is the energy of a photon of green light of wavelength 515 nm.
Generally the equation for the energy of a photon is mathematically given as Therefore the energy of a photon of green light with a frequency of 580 x 1014s is. 10 When a photon of light is absorbed by an atom in a chlorophyll molecule. Hospital X-ray generators emit X-rays with wavelength of about 150 nanometers nmnm where 1nm109m1nm109m.
Where h is Plancks constant v is the frequency of photon. What is the energy of a photon of green light. Its what gives cells their energy.
E 19878 x 1028 650109. What is the energy of a photon of green light having the frequency of 580x 10x 14 Hz. Therefore we can rewrite the above constant for hc in terms of eV.
398x10-19J Ehν 6626x10-34Js 600x1014s-1 C. What is the energy of a photon of the X-rays. Hc 199 10 -25 joules-m 1ev1602 10 -19 joules 124 10 -6 eV-m.
How do you calculate the energy of one photon of light. The energy of a single photon of green light of a wavelength of 520 nm has an energy of 238 eV. When photon light touches the skin it is absorbed as a form of energy in the cells.
Once this energy is absorbed in the body it forms nitric oxide. 11 What color of light in the visible spectrum appears. E 454 x 10¹⁹ J.
What is the energy in joules of 10 mol of photons of green light. E 663 10³⁴ Js x 685 X 10¹⁴ s¹. 7 Does visible light have high or low energy.
8 Which one of the following has the highest energy. The speed of light has a value of approximately 300 108 m s The wavelength should have units of meters. What is the energy in joules of ONE photon of green light.
If you wish to you may calculate this for yourself. The unit of frequency is f frac1mathrm s though this is noted somewhat more compactly as frac1mathrm s mathrmHz. Energy of photon E hv.
7 Does chlorophyll absorb green light. Keep in mind that the shorter the wavelength the more energetic the radiation. Light with a wavelength of 525 nm is green.
All frequencies of visible light will have an energy in the 10 19 J range of values. ATP is essential for cellular functioning. What is the energy in joules of 10 mol.
The energy associated with a single photon is given by E h ν where E is the energy SI units of J h is Plancks constant h 6626 x 10 34 J s and ν is the frequency of the radiation SI units of s 1 or Hertz Hz. The energy is 3843110e-14 joule. Photon energy formula is given by E hc λ.
6 Which light has highest energy in vibgyor. Let Plancks constant h 4136 10 15 eV s and the speed of light c 300 10 8 ms. 9 At what wavelengths of visible light does chlorophyll absorb the greatest amount of light energy.
Chapter 7 Problem 5PS is solved. What is the energy in joules of ONE photon of green light. X 6626 x 10 34 J s 576 x 10 14 s 1 x 382 x 10 19 J Comment.
The green light has a frequency of 566 1014H z. The frequency should have units of H z or s1. 9 What color is the visible light spectrum.
The energy of a particular color of green light is 382 x 10. 10 Which has a higher energy blue light or green light. Green light has a wavelength of 50 x 102 nm.
8 What is the wavelength of chlorophyll A. The energy in Joules of one photon as.

The Energy Of A Photon Of Green Light Of Wavelength 5000 A O Is
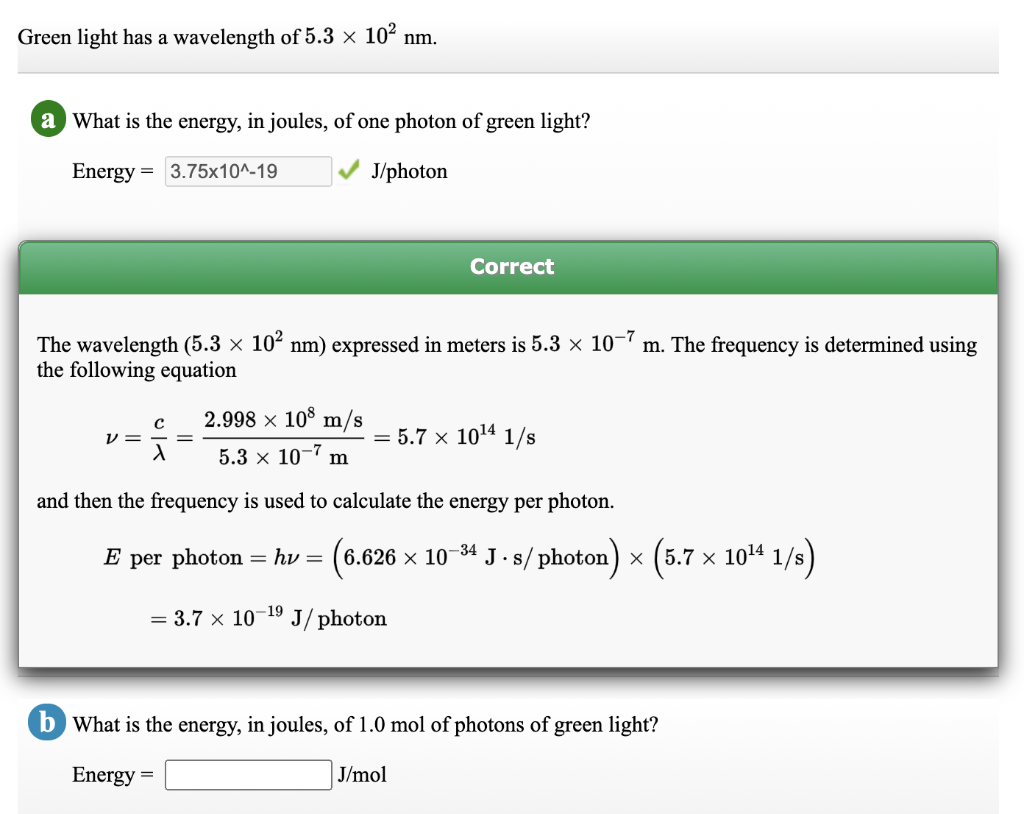
Solved Green Light Has A Wavelength Of 5 3 X 102 Nm A What Chegg Com

Sunlight Photon An Overview Sciencedirect Topics

Green Light Has A Frequency Of About 6 00 X 10 14 S 1 What Is The Energy Of A Photon Of Green Light Home Work Help Learn Cbse Forum

Photon Flux Density An Overview Sciencedirect Topics

Green Light Has A Frequency Of About 6 00 X 10 14 S 1 What Is The Energy Of A Photon Of Green Light Home Work Help Learn Cbse Forum

Suppose 10 17 J Of Energy Is Needed By The Interior Of Human Eye To See An Object How Many Photons Of Green Light 1 550 Nm Are Needed To Generate This

What Is The Number Of Photons Of Light With A Wavelength Of 4000 Pm That Provide 1 J Of Energy

How To Calculate Energy Of A Photon

What Exactly Is A Photon Quantum Laser Lights Physics

Beauty Massager 6 In 1 White Facial Therapy Skin Rejuvenation Acne Scar Removal

For Some Reason I Never Posted These Notes So Here You Go Today I Ve Mostly Studied Math Cross Pro Apuntes De Clase Como Tomar Apuntes Libreta De Apuntes

Green Light Has A Frequency Of About 6 00 X 10 14 S 1 What Is The Energy Of A Photon Of Green Light Home Work Help Learn Cbse Forum

Solar Panels From Cpt Break Efficiency Barrier Cleantechnica

7 Colour Led Light Therapy Mask In 2022 Light Therapy Mask Led Light Therapy Mask Led Facial

Energy Momentum Of A Photon Equation Calculations Video Lesson Transcript Study Com